The amphipod Hyallela azteca and the Asian clam Corbicula fluminea are commonly found in water bodies throughout the southeastern United States (McMahon 1991) (Pennak 1989). Amphipods are opportunistic feeders that consume animal and plant material, they rarely attack live prey but readily consume freshly killed animals (Pennak 1989). Amphipods should be able to utilize dead clam material available periodically in natural systems however monocultures of clam populations held under laboratory conditions may present a unique opportunity for amphipods due to the elimination of predators, ability to utilize clam material as a food source and the lack of competition for dead clam material. Invasion of live clams is a previously undescribed behavior for Hyallela azteca however recent observations made in a outdoor laboratory monoculture of Corbicula fluminea demonstrate the ability of amphipod to successfully insert themselves into the body cavities of live clams, utilize dead clam material, possibly aid in altering clam shell margins and may influence clam population mortality.
The above observations were made at an outdoor research facility constructed at the University of Florida Dairy Research Unit in Hague, Florida. This is a recirculating system consisting of a reservoir pond that supplies water to raceways. The raceways contain a coarse sand substrate and stocked with Corbicula at densities of approximately 1400 clams per m2. Amphipods began appearing in dead clams removed from the raceways on July 26, 2002 (38 days after introduction of clams). Several live clams on the substrate surface were removed for inspection on yielding clam with a 4 mm long x 2 mm wide, elliptical notch spanning both valves along the posterior shell margin at the siphon region. Amphipods were also observed emerging from the notches in the closed shell valves of the clams after a few minutes out of the water. The clam was immediately shucked to reveal six additional live amphipods enclosed in the shell cavity.
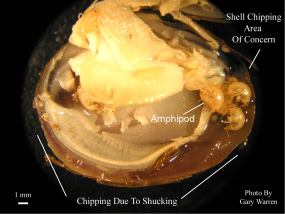
Figure 1
Amphipods Found in a Live, Notched Clam
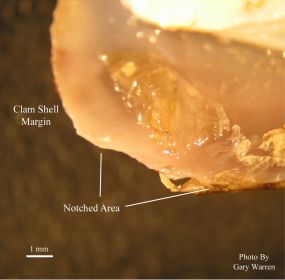
Figure 2
Chipping In The Notched Area of Shell
Judging from these observations, amphipods may be exhibiting a parasitic behavior when introduced to clam monocultures. The ability of the amphipod to successfully parasitize a living clam over a long period of time is probably inhibited by the amphipod’s nutritional demands and large body size. Unlike other much smaller parasites such as mites and other microorganisms often found in freshwater mussels that rely on the host for its entire life cycle and consume minimal quantities of live clam material (Fisher et al 2000) (Dimock pers. comm. 2002), a population of amphipods may require a considerable amount of clam biomass as a food source. Soft tissue such as gill, palp, mantle and foot organs would be potential targets for amphipods inside the shell cavity and destruction of these tissues may limit the ability of the clam to perform important functions including respiration and feeding. Amphipods living inside the shell cavity may also be able to consume clam excrements, however a population of amphipods may still take up a considerable amount of space inside the shell cavity which may inhibit the ability of the clam to successfully move water necessary for filter feeding and respiration. Declines in clam health due to destruction of soft tissue and restriction of water movement by amphipods may be contributing to clam mortality in monoculture systems.
The mode of introduction of amphipods into live clam body cavities has not been observed directly, however it is unlikely that amphipods are introduced through the incurrent siphon in a juvenile form and mature inside the shell. Amphipod molting takes place every 3 to 40 days depending upon conditions (Pennak 1989) and the lack of molted carapaces found within the clam body cavities is consistent with an invasion by adult amphipods rather than introduction and growth of juveniles unless carapaces were consumed by the adults or ejected by the clam. Assuming that the adult amphipods are invading the clams then one must consider an alternate mode of introduction used by the amphipods and the importance of the formation of shell notching.
Amphipods may be small enough to enter through one of the clam siphons however tentacles on the rim of the siphons may act as sensory organs that cause the clam to retract siphon and foot organs or “clam up” in response to touch. Clams utilize shell valve opening and closing in order to facilitate filter feeding (Lauritsen 1985) and are able to expel particles from the shell margins under normal conditions, however the rapid closure response to touch may cause the entrapment of sediment particles between the opposing shell margins. Depending on the size and hardness of the particle and extent of penetration of the shell cavity one either the valves will be become lodged open or the pressure applied by the adductor muscles will overcome the strength of the shell in that area causing small chip will be formed with the newer, thinner tissue along the shell margin being especially vulnerable. The following explanations are offered to describe the introduction of amphipods into live clams and consequent formation of these shell notches.
One explanation is that a passing amphipod on the sediment surface can trigger a premature retraction of the foot in response to touch and trap a sediment particle between valves upon closing. It is important to note that lodging of sediment particles between shell margins has been observed in both river and raceway sediments as a result of hand-excavation in earlier collections. If the resultant particle diameter were large enough to allow an amphipod to enter the shell cavity then this would initiate the invasion and explain the amphipods present in the buried clam with no shell notching. Shell notching may then occur as a result of the clam attempting to expel the amphipods by opening and closing the valves and use of the foot organ to dislodge them. Repeated attempts to remove the amphipods and the lack of foot organ present in the siphon region may have lead to chipping in the softer shell margin and consequent formation of the shell notches observed. The clam then migrates to the sediment surface as a response to declining health.
Another possible explanation is that repeated rapid closure events during periods of clam feeding causes chipping and eventual notch formation in the shell. Notches form at the siphon region as mentioned above due to restrictions of the extension of the foot organ. When the notches become large enough, amphipods then invade the clam. As a response to declining health caused by the presence of amphipods, the clam then migrates to the substrate surface.
In both explanations the presence of amphipods is assumed to be a negative impact causing clam migration to the substrate surface in response to declining health. This assumption that clam migration is associated with clam health is consistent with previous observations of clams in raceway experiments that show the same migration pattern before death without the presence of amphipods or shell notching.
Clam health can also be adversely affected by environmental factors such as temperature, pH, ammonia and lack of food. Prolonged exposure to unfavorable environmental conditions and consequent decline in health may make clams more susceptible to amphipod invasion because unhealthy clams may respond slower to touch therefore allowing more time for an amphipod to penetrate the shell cavity. These clams may also lack the energy resources to expel amphipods from the shell cavity.
Introduction of fish to these systems may reduce amphipod abundance but may also affect clam population recruitment due to predation stress on juvenile clams. Clams smaller than 7mm in shell length are the most susceptible to predation due to their lack of shell strength and are preferred by most molluscivorous fish (McMahon 1991). Adult Hyallela azteca range in size from 4mm to 8mm (Pennak 1989), a characteristic that may classify both organisms as potential prey for a variety of common fish species including sunfish, carp and catfish. A smaller species of fish such as mosquitofish (Gambusia affinis) or sailfin molly (Poecilia latipinna) may reduce the risk of clam predation while reducing amphipod populations depending upon their ability to crush the immature clam shell and feeding selectivity for amphipod prey.
The other potential option for controlling amphipod invasion may be the use of a different substrate material. Corbicula are able to utilize a variety of substrates from fine sand to gravel (Belanger et al 1985) (Blalock & Herod 1999) and well-point filtration sand was chosen for use at the Hague system because of its intermediate particle sizing and low cost. One solution may be the selection of a finer grade sand because the smaller grain size would limit the distance that the shell valves would remain open if a particle were to become wedged, possibly reducing the chances of amphipods entering the clam shell cavity. Finer sand may also promote the establishment of Corbicula in natural systems (Belanger et al 1985) but may also leave juveniles susceptible to fish predation. Another solution may be to use a fine grade of smooth river gravel. The larger grain size may limit shell chipping because the aggregates are too big to become wedged between shell valves. The gravels may also provide juvenile clams with adequate refuge from fish predators intended for amphipod control however the larger particle size may limit mobility of adult clams, causing undue stress from excess energy expenditures.
References
Belanger, S.E., et al. 1985. Sediment preference of the freshwater Asiatic clam, Corbicula fluminea. The Nautilus. Vol 99(2-3), pp 66-72.
Blalock, H.N. and J.J. Herod. 1999. A comparative study of stream habitat and substrate utilized by Corbicula fluminea in the New River, Florida. Florida Scientist
Dimock, Jr., R.V. 2002. Personal communication. Professor, Wake Forest University Biology Dept. Winston-Salem North Carolina.
Fisher, Ginger R., et al. 2000. The symbiotic water mite Unionicola Formosa (Acari: Unionicolidae) ingests mucus and tissue of its molluscan host. Journal of Parasitology. pp 1254-1258.
Lauritsen, D.D. 1985. Filter-feeding, food utilization, and nutrient remineralization by Corbicula fluminea (Bivalvia) and its contribution to nutrient cycling in a North Carolina river. PhD Thesis, NC State University. 129 pp.
McMahon, R.F. 1991. Mollusca: Bivalvia. Pages 315-399 in: Thorpe, James H. and Alan P. Covich editors. Ecology and classification of North American freshwater invertebrates. Academic Press, New York.
Pennack, R.W. 1989. Freshwater invertebrates of the United States, Protozoa to Mollusca. Wiley, New York. 628 pp.
Sinclair, R.M. and B.G. Isom. 1963. Further studies on the introduced Asiatic clam Corbicula in Tennessee. Tennessee Pollution Control Board, Tennessee Dept of Public Health. Nashville, Tennessee. 76 pp.
Warren, Gary. 2002. Personal communication. Florida Fish and Wildlife Commission Biologist. Gainesville, FL.